Microneedling with CBD
A Clinical Study for Acne Relief
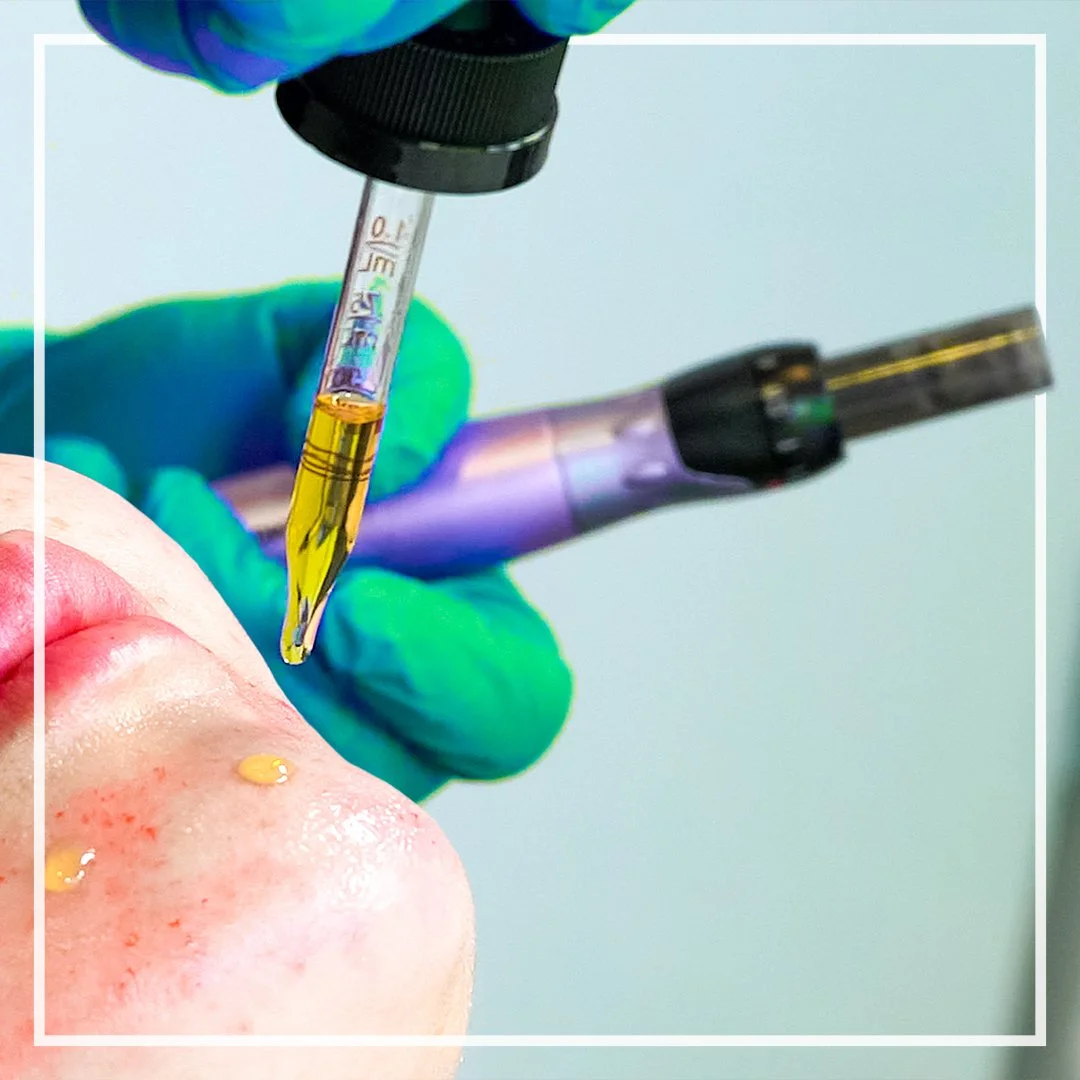
Kian Karimi MD, FACS, is recruiting volunteers with moderate to severe acne of the face, for a clinical study evaluating the effectiveness of microneedling with topical cannabidiol (CBD) in reducing inflammation, acne-causing bacteria, and improving overall skin health.
Eligible participants will receive three (3) treatment sessions of collagen induction therapy (CIT), also known as microneedling, with topical application of cannabidiol oil (HealMD Topical), conducted over nine (9) weeks in Los Angeles, CA.
STUDY PARTICIPATION
Who may participate in this study?
You may qualify for this research study if the following applies to you:
Male or non-pregnant, non-lactating female, twenty-two (22) years of age or older.
Females of childbearing potential must agree to the use of a reliable method of contraception throughout the study.
Have a Global Acne Severity Scale Score of 3 (Moderate), 4 (Severe), or 5 (Very Severe).
Have no plans to begin a new skincare routine or medical treatment program (Isotretinoin, Accutane) through the course of the study.
Willing to abstain from any aesthetic or surgical procedure in the treatment area for the duration of the study.
Have not been on Isotretinoin (Accutane) treatment for at least six (6) months prior to the start of the study.
You do not have significant history or current evidence of any uncontrolled chronic or serious disease or medical condition that would, in the judgment of the investigator, would put the subject at undue risk or compromise the study assessments.
You are not an employee of the Investigator or research center or their immediate family members.
Inability to understand the requirements of the study and the relative information and are unable or not willing to comply with the study protocol.
You are not currently taking Isotretinoin (Accutane), and have not taken Isotretinoin (Accutane) within six (6) months.
You have not used topical or oral therapies that include benzoyl peroxide, azelaic acid, salicylic acid, and hydroquinone thirty (30) days prior to study entry.
You do not have exposure to any other investigational drug/device within thirty (30) days prior to study entry.
You do not have sunburned at time of anticipated treatment. Subject must also be willing to avoid significant sun exposure throughout participation.
You have not had recent facial plastic surgery, aesthetic treatment, or dermatological treatment at treatment sites that would interfere with ability to receive dermal infusion treatment (microneedling).
You do not have facial hair that would interfere with the visualization of treatment sites.
You do not have abnormal vision assessments.
You are not known to be noncompliant or are unlikely to comply with the requirements of the study protocol (e.g. due to alcoholism, drug dependency, mental incapacity) in the opinion of the investigator.
There are other eligibility requirements. A final decision on whether this clinical trial is suitable for you will be made after you speak with the study doctor at the research site.
Your next steps
See if the study may be suitable for you by reviewing the criteria above
Submit your contact details to study personnel
Be contacted by a study coordinator for further information